The Contract Research Organization (CRO) landscape in India is undergoing a remarkable transformation, driven by the global demand for clinical research and the need for innovative healthcare solutions. As the pharmaceutical and biotechnology industries continue to evolve, CROs are positioned to play a pivotal role in this growth, particularly in India. This article explores the future of CRO in India – Cliniexperts, focusing on emerging trends, challenges, and the opportunities that lie ahead.
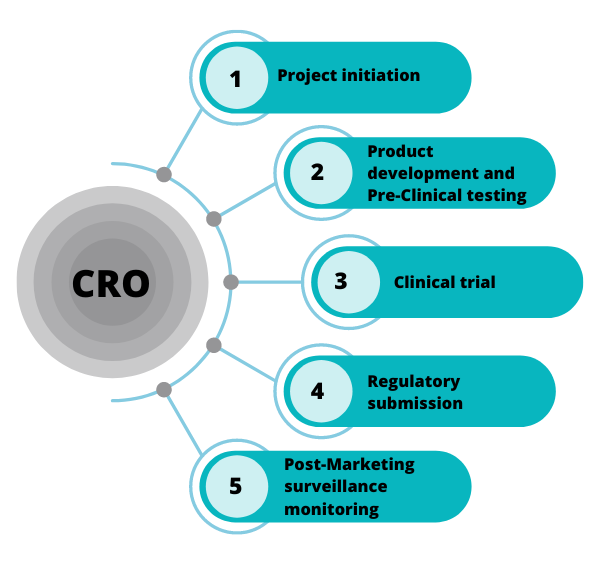
The Current State of CROs in India
India has emerged as a prominent player in the global CRO market due to its vast patient population, skilled workforce, and cost-effective services. The country has become a hub for clinical trials, attracting both domestic and international pharmaceutical companies. As of now, India hosts a myriad of CROs, ranging from full-service organizations to niche players specializing in specific therapeutic areas.
Key Factors Driving Growth
- Regulatory Reforms: The Indian government has taken significant steps to streamline the regulatory framework governing clinical trials. Initiatives aimed at reducing approval times and enhancing transparency are making India a more attractive destination for clinical research.
- Rising Demand for Innovative Therapies: With the increasing prevalence of chronic diseases and the need for personalized medicine, there is a growing demand for innovative therapies. CROs are essential in facilitating the development of these treatments, making them a vital component of the healthcare ecosystem.
- Technological Advancements: The integration of technology in clinical research is transforming how CROs operate. Tools such as electronic data capture (EDC), telemedicine, and artificial intelligence (AI) are enhancing efficiency and improving data quality.
Future Trends in CROs in India
As we look ahead, several trends are expected to shape the future of CROs in India:
1. Increased Focus on Patient-Centric Trials
Patient-centricity is becoming a key focus in clinical research. CROs will increasingly prioritize patient engagement, using innovative recruitment strategies and tools to enhance participant retention. Involving patients in the design and execution of clinical trials will lead to more relevant and effective studies.
2. Adoption of Digital Health Technologies
The future of CROs will be significantly influenced by the rise of digital health technologies. Remote monitoring, wearable devices, and mobile health applications will enable real-time data collection and patient monitoring. This shift will not only enhance trial efficiency but also improve patient outcomes.
3. Emphasis on Real-World Evidence (RWE)
As regulatory authorities recognize the importance of real-world evidence, CROs will play a critical role in generating and analyzing RWE. By leveraging data from everyday clinical practice, CROs can provide insights into treatment effectiveness and patient experiences, supporting regulatory submissions and market access strategies.
4. Expansion of Therapeutic Areas
The scope of CRO services is expected to expand beyond traditional therapeutic areas. As India’s healthcare landscape evolves, CROs will increasingly engage in research related to personalized medicine, biologics, gene therapy, and rare diseases. This diversification will open new opportunities for CROs and enhance their relevance in the market.
5. Global Collaborations and Partnerships
Collaboration between Indian CROs and global pharmaceutical companies will continue to grow. As international sponsors seek to conduct trials in India, local CROs will need to strengthen partnerships and establish networks to facilitate these collaborations. This trend will foster knowledge exchange and enhance the overall quality of clinical research.
Challenges Ahead
While the future of CROs in India looks promising, several challenges must be addressed:
- Talent Shortage: The demand for skilled professionals in the clinical research field is growing. However, there remains a shortage of trained personnel, which can hinder the growth of CROs. Investment in training and development will be essential to build a competent workforce.
- Regulatory Complexities: Despite recent reforms, navigating the regulatory landscape in India can still be challenging. CROs must stay abreast of changes in regulations and ensure compliance to avoid delays in trial approvals.
- Competition: The increasing number of CROs in India has intensified competition. To remain competitive, organizations must continually innovate, enhance their service offerings, and maintain high standards of quality.
Conclusion
The future of CROs in India is bright, characterized by growth, innovation, and increased relevance in the global healthcare ecosystem. With the demand for clinical research services on the rise, CROs will play a crucial role in advancing drug development, improving patient outcomes, and enhancing the overall quality of healthcare.
CROs like CRO in India – Cliniexperts are at the forefront of this transformation, leveraging local expertise and global best practices to shape the future of clinical research in India. By embracing emerging trends and addressing challenges, Indian CROs can position themselves as leaders in the global CRO market, contributing to the advancement of healthcare solutions worldwide.


Leave a Reply